Compounding practices encompass all of the functions necessary to create a nonsterile compound including selecting storing compounding dispensing the mixture and monitoring the patient. Quality Concerns While the importance of extemporaneous compounding of dermatologicals is well established 2 5 17 18 the benefits of the products to patients are obtained provided that.
Many patients need drug dosages or strengths that are not commercially available.

. Question 11 Who provides the final product check in the compounding process. The need for a quality assurance system is well documented in USP chapters see Compounding Controls under Good Compounding Practices 1075. A This regulation describes the requirements of minimum current good compounding practice for the preparation of drug products by pharmacies or other facilities with permits issued by the Arkansas State Board of Pharmacy.
Compounding includes the. Addresses quality issues in compounding Includes standards regarding ingredient selection. A the USP b pharmacist c pharmacy inspector d the FDA.
An increased effort to regulate the compounding practice is necessary as the practice is found to be expanding slowly in the countrys public hospitals. Based on 23 documents. Compounded drugs are not FDA-approved.
This chapter deals with some of the practical aspects of dispensing concentrating on the small-scale manufacture of medicines from basic ingredients. A compounding hood is a large piece of equipment that prevents particles from entering and leaving a work area like a complex version of the vent fan above a cooking stove. Quality Control and Verification under Pharmaceutical Compounding.
A quality assurance program is a system of steps and actions taken to ensure the maintenance of proper standards in compounded preparations. CURRENT GOOD COMPOUNDING PRACTICES 1. Current good compounding practices means the minimum standards for methods used in and facilities or controls used for compounding a drug to ensure that the drug has the identity and strength and meets the quality and purity characteristics it is represented to possess.
Compounding pharmacies are also encouraged to adopt standards for pharmaceutical calculations quality control and equipment. Select the true statements regarding good compounding practices GCP. Based on a history of the pharmacist receiving valid orders.
The purpose of this chapter is to provide compounders with guidance on applying good compounding practices for the preparation of compounded formulations for dispensing andor administration to humans or animals. Hoods are mostly used. Equipment used in compounding needs to be appropriate for use of sufficient size and maintained as directed by the manufacturer.
States are encouraged but not mandated under federal guidelines to adopt USP chapters on sterile compounding chapter 797 nonsterile compounding chapter 795 and handling hazardous drugs in health care settings. AR State Board of Pharmacy Regulations BReg 07-02-0002. Many patients need dosage forms such as suppositories oral liquids or topicals that are not commercially available 3.
This chapter is intended to provide information as a supplement to other relevant chapters. The equipment or utensils used for compounding of a drug preparation USP29 shall be of appropriate design and capacity. For meds that have a short expiration date once liquid or diluent is added to their powder form.
The purpose of this chapter is to provide compounders with guidance on applying good compounding practices for the preparation of compounded formulations for dispensing andor. Based on the unsolicited receipt of a valid prescription. Many patients are allergic to excipients in commercially available products 4.
By a licensed pharmacist or a physician. Drug compounding is often regarded as the process of combining mixing or altering ingredients to create a medication tailored to the needs of an individual patient. The equipment should USP29 be stored in such a manner as to protect it from contamination and shall be located in such a place as to facilitate operations for its use maintenance and cleaning.
Mechanical electronic and other types of equipment used in compounding shall be routinely inspected calibrated as necessary and checked to ensure proper. - Good Compounding Practices. Good compounding practices regulate which aspect of equipment used.
Act of reducing particle size of a solid using a mortar and pestle. For an identified individual patient. Compounding Laws and Policies.
A size b year made c storage d labeling. Raw ingredients and finished products do not undergo Generally the current compounding practice status for evaluation for quality aspects like purity particle size dermatologicals in the public hospitals indicated the need viscosity potency stability and other attributes that are for increased efforts on the regulation of the practice and appropriate to demonstrate products. The following discussion is applicable to.
Ted quantities may be compounded prior to receiving a prescription. This means that FDA does not review these drugs to evaluate their safety effectiveness or quality before they reach patients. Understand the role of compounding Pharmacists dispensing in practice Able to resolve problems of making preparations-specific preparations Applying the techniques of solving problems related to the administrative aspects and clinical farmasetis in the giving of drugs on patients Apply the dose determination based on the.
Good compounding practices regulate which aspect of equipment used. Tech calculates quantity and adds a specific amount of liquid to a container prefilled with powdered drug. Often used before mixing a solid w a liquid.
This process is called compounding or extemporaneous dispensing. Additionally good practice which applies to all aspects of dispensing will be considered.
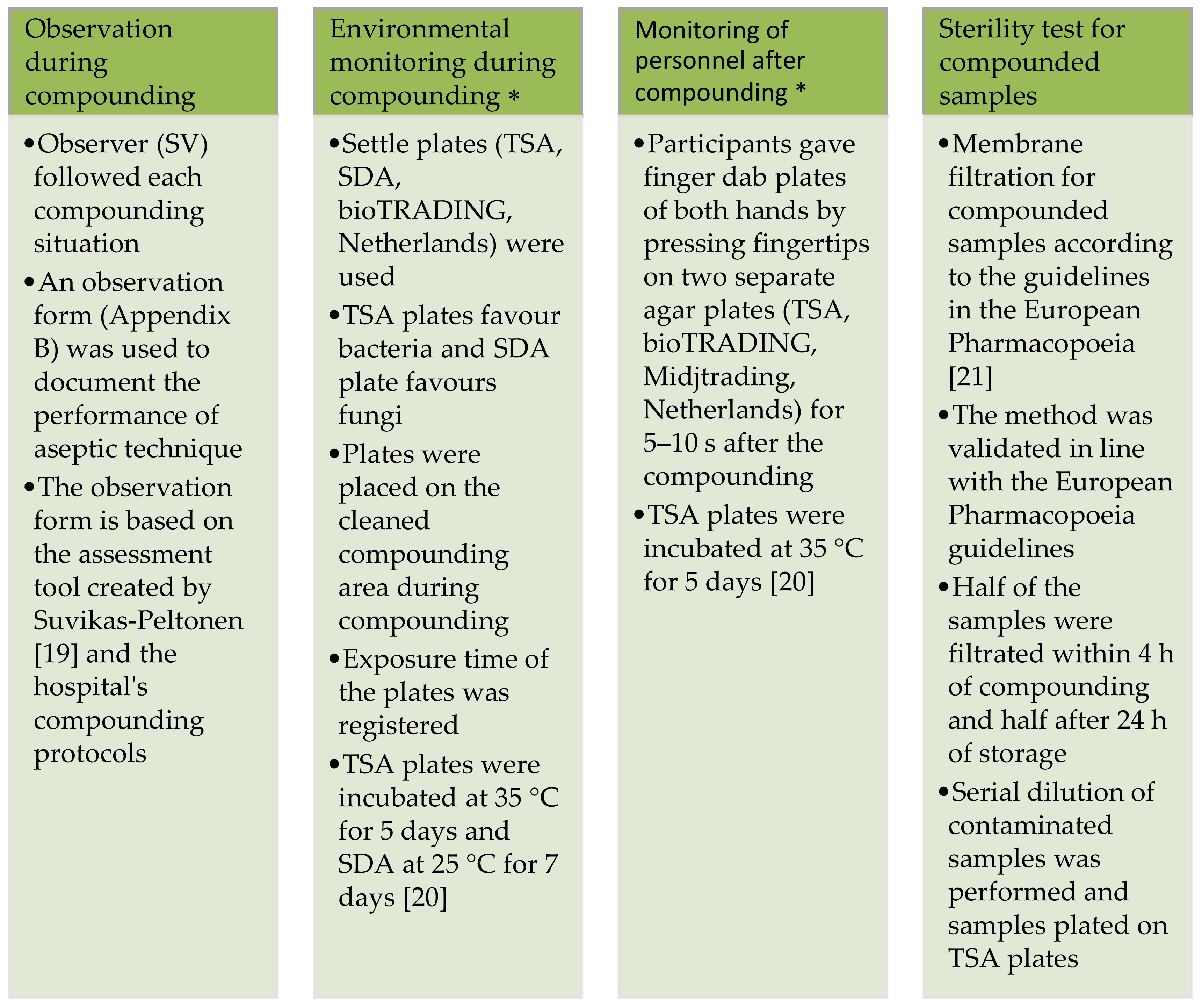
Healthcare Free Full Text Compounding Parenteral Products In Pediatric Wards Effect Of Environment And Aseptic Technique On Product Sterility Html
Policies And Procedures For Cleaning Engineering Controls November 2018 Pharmacy Purchasing Products Magazine

Trends In Regulatory Actions For 503b Compounders December 2019 Pharmacy Purchasing Products Magazine
Trends In Regulatory Actions For 503b Compounders December 2019 Pharmacy Purchasing Products Magazine
0 Comments